
#10,417
Seven months ago, in H5N1 Detected In Swan Die Off In Henan Province, I wrote about the discovery of nearly 100 dead birds (swans & ducks) at the Sanmenxia reservoir in Henan province, which authorities attributed to the H5N1 virus. Sanmenxia reservoir is a major overwintering habitat for a variety of waterfowl migrating between China, Mongolia, and Siberia.
We take special note of these large die offs because in the past they have occasionally denoted a change in the virus, as was the case in Qinghai Lake both in 2005 and 2009.
- What emerged at Qinghai Lake in 2005 was clade 2.2 (aka QH05) of the H5N1 virus. And over the next 18 months, this new clade vastly expanded its geographic range across Asia, and into Europe and Africa (see H5N1 Influenza Continues To Circulate and Change 2006 by Webster et. al.).
- Four years later researchers found evidence of another clade (2.3.2) (see 2011 EID Journal New Avian Influenza Virus (H5N1) in Wild Birds, Qinghai, China), at another bird die off in the same region. In short order the 2.3.2 clade began to show up in migratory birds, and poultry, from Japan to India, supplanting the old 2.2 clade in many regions.
Earlier this summer, in Deja Flu: Another Qinghai Lake H5N1 Die Off, we saw another reported mass die off of waterfowl, purported to be due to H5N1, but we’ve not seen any analysis come out of that incident yet.
Earlier this week, however, Scientific Reports published an open access study on the genetics of the H5N1 virus that caused last January’s die off in Henan Province, and once again we find ourselves looking at a novel reassortment.
First the abstract (the entire study is available online) then I’ll return with a bit more.
Highly Pathogenic Avian Influenza A(H5N1) Virus Struck Migratory Birds in China in 2015
Yuhai Bi,a,1,2,6 Zhenjie Zhang,3 Wenjun Liu,1,6 Yanbo Yin,5 Jianmin Hong,7 Xiangdong Li,8 Haiming Wang,9 Gary Wong,1 Jianjun Chen,6,10 Yunfeng Li,11 Wendong Ru,11 Ruyi Gao,11 Di Liu,1,6 Yingxia Liu,2 Boping Zhou,2 George F. Gao,1,2,6,12 Weifeng Shi,b,3 and Fumin Leic,4,6
Abstract
Approximately 100 migratory birds, including whooper swans and pochards, were found dead in the Sanmenxia Reservoir Area of China during January 2015. The causative agent behind this outbreak was identified as H5N1 highly pathogenic avian influenza virus (HPAIV). Genetic and phylogenetic analyses revealed that this Sanmenxia H5N1 virus was a novel reassortant, possessing a Clade 2.3.2.1c HA gene and a H9N2-derived PB2 gene.
Sanmenxia Clade 2.3.2.1c-like H5N1 viruses possess the closest genetic identity to A/Alberta/01/2014 (H5N1), which recently caused a fatal respiratory infection in Canada with signs of meningoencephalitis, a highly unusual symptom with influenza infections in humans. Furthermore, this virus was shown to be highly pathogenic to both birds and mammals, and demonstrate tropism for the nervous system.
Due to the geographical location of Sanmenxia, these novel H5N1 viruses also have the potential to be imported to other regions through the migration of wild birds, similar to the H5N1 outbreak amongst migratory birds in Qinghai Lake during 2005. Therefore, further investigation and monitoring is required to prevent this novel reassortant virus from becoming a new threat to public health.
(Continue . . . )
As we’ve discussed before, since HPAI H5N1 emerged as a single clade (0) in China in 1996, it has has evolved into more than 20 clades and subclades – and many more variants - and more are to be expected. This constant evolution is something we’ve looked at often, including Moving Viral Targets & EID Journal: The Expanding Variants Of H5N1).
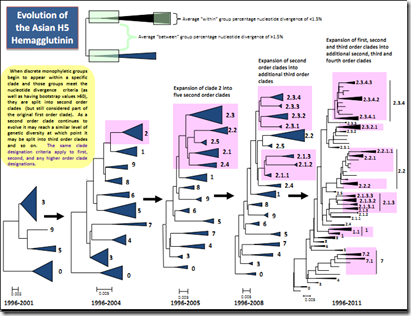
Not all of these clades are currently circulating
While some of these evolutionary variations come about slowly due to antigenic drift, more abrupt changes can come about through antigenic `shift’ – or reassortment. Which is the process that produced the novel virus described in today’s report.
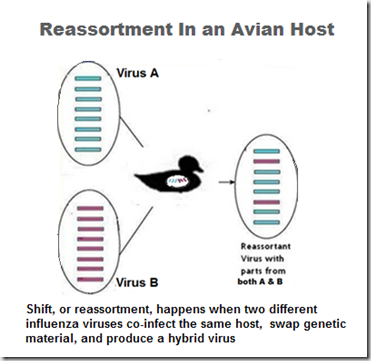
The H5N1 viruses that are circulating in China can be genetically quite distinct from the viruses circulating in Egypt, or Vietnam, or Indonesia. Some regions may have multiple clades in circulation, and some clades may pose more of a risk to humans than others (see Differences In Virulence Between Closely Related H5N1 Strains).
Since clades are based on the HA gene of the flu virus, we can see several different subtypes (H5N1, H5N8, H5N2) fall into the same clade.
The novel reassortment in today’s is a mix of a H5N1 clade 2.3.2.1c virus and an H9N2-derived PB2 gene.
Clade 2.3.2.1c describes an H5 HA gene segment which has been showing up in Vietnam, China, India, Bulgaria, and Indonesia for several years, and was recently detected in Nigerian poultry (see EID Journal: H5N1 In Nigerian Poultry – 2015.
Of note, this is the same H5 clade isolated from a Nurse who returned to Alberta, Canada from a trip to China (see Alberta Canada Reports Fatal (Imported) H5N1 Infection) in late 2013, and similar to one that killed a captive tiger in Jiangsu Province - also in 2013 - with both cases exhibiting unusual neurological symptomology.
To close, a few excerpts from the discussion section of the report.
The novel isolates were found to be highly pathogenic to chickens and mice, and virus isolated from mouse brains after challenge. It should be noted that meningoencephalitis was also observed in the fatal human case with Alberta2014, an unusual outcome for infections with H5N1 HPAIV in humans20. Neurological symptoms were also noted in the non-surviving tiger infected with Tiger2013, with the heart, liver, spleen, lung, kidney, aquae pericardii, and cerebrospinal fluid all positive for H5N1 virus as detected by real-time RT-PCR21. This suggests that the novel Sanmenxia Clade 2.3.2.1c-like H5N1 viruses possesses tropism for the nervous system in several mammal species, and could pose a significant threat to humans if these viruses develop the ability to bind human-type receptors more effectively.
(SNIP)
It is believed that overlapping migratory flyways help circulate H5 HPAIVs amongst different bird species, and allow the spread of the virus across continents8. Recently sequential outbreaks of the H5N8 virus in domestic poultry in China, Korea, and Japan during 2010–201534,35, Europe in 2014–201536, and North America in 2014–20154,37,38 were considered to have occurred due to waterfowl migration36,37,39. Therefore, the migration of wild birds plays an important role in the transmission and spread of H5 HPAIVs, posing a severe risk to animal and human health. Further investigation is required to monitor the evolution and transmission of these novel Sanmenxia Clade 2.3.2.1c-like H5N1 isolates, in order to prevent their spread to other countries and avoiding a repeat of what had happened in the past with circulating Qinghai-like Clades 2.2 and 2.3.2 H5N1, and Clade 2.3.4.4 H5N8 viruses7,9,15,16,19,36,37,40,41.






















 src="https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEh4zgoKkY5esDyGDfXmhp5tz0W8H2jEgsRJx2wm9317hpr6CTdO8i4DPQj5mF-OAprw6GVcNt84Pt9Yp5U6XEz5h_pAP7azclFEO7kSUzDjr31IvLdzT01usqHnjVk1bBWsqpHQX6G4AIU/s1600/Photo0783.jpg" />
src="https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEh4zgoKkY5esDyGDfXmhp5tz0W8H2jEgsRJx2wm9317hpr6CTdO8i4DPQj5mF-OAprw6GVcNt84Pt9Yp5U6XEz5h_pAP7azclFEO7kSUzDjr31IvLdzT01usqHnjVk1bBWsqpHQX6G4AIU/s1600/Photo0783.jpg" />












